Recent Activity


Data Partners & Collaborators
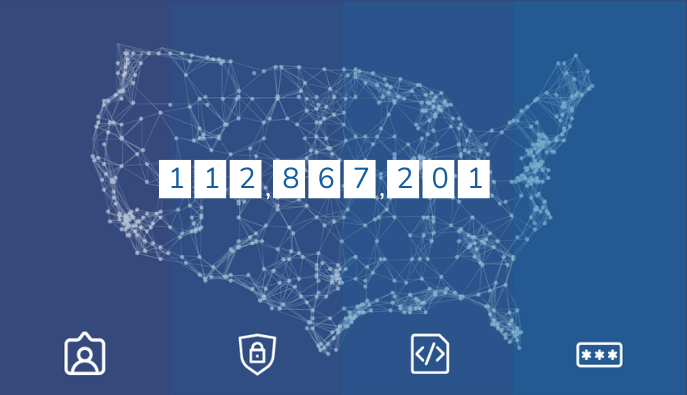
The FDA gets data for the Sentinel System through its Data Partners. The Data Partners are the ones who access, maintain, and protect the data in a distributed system. The Sentinel Operations Center partners with a network of collaborators. This includes Data Partners and Collaborating Institutions. Together, they provide healthcare data and scientific, technical, organizational expertise.

1. Community Building and Outreach Center (CBOC)
2. Innovation Center (IC)
3. Sentinel Operations Center (SOC)
These centers collaborate to advance regulatory science using the Sentinel System. However, each center operates independently and has a dedicated leadership team and staff. The three centers each carry out projects associated with their strategic objectives.

